General Effects Of Crack Use Include Burning The
- General Effects Of Crack Use Include Burning Of The
- General Effects Of Crack Use Include Burning The Body
Abstract
The fleeting high from smoking crack can be outweighed by a host of negative effects. Though these can vary as widely as the positive effects listed above, commonly reported side effects include: Irritability. Aggressive, paranoid behavior. Abdominal pain. Of course, one of the major effects of crack cocaine is rapid development of addiction. Cocaine causes the strongest mental dependency of any drug. This dependency can occur within just days of starting to use crack cocaine. Binge use of crack cocaine can result in a psychotic, over-stimulated state accompanied by paranoia and compulsive behavior.
Reviewed available studies of the impact of fetal cocaine exposure on child medical and developmental outcome, as well as the current status of clinical psychological interventions and research strategies. Current studies are inconclusive but suggest that prenatal exposure to crack-cocaine can have significant effects on the growth and neurological development of the infant, with the potential of later learning and behavioral disabilities. Social-environmental correlates of maternal cocaine use are confounding factors with known negative effects on child outcome. Large, population-based studies using multivariate analyses are needed to determine the independent effects of cocaine on child outcome relative to other confounding variables.
Psychotropic drug use during pregnancy may have adverse effects on the mother, her pregnancy, the developing fetus, newborn, and growing child. Some drugs, such as heroin, adversely affect reproductive outcome and fetal growth, while others, such as alcohol, are fetal teratogens, producing congenital anomalies of the heart and central nervous system with subsequent mental retardation (Finnegan, Chappel, & Kreek, 1982). The greatest increases in drug use popularized in the late 1970s and accelerating into the 1990s have been among young adults of child-bearing age using cocaine (). As a result, new casualties of the recent “crack” cocaine epidemic have been primarily pregnant women and their children. Maternal use of cocaine during pregnancy has risen to affect 10–15% of the populations of urban neonatal nurseries and intensive care units in major cities in the United States (; ; ).
The number of infants born exposed in utero to cocaine has increased over the last 5 years. The National Hospital Discharge Survey identified 13,765 infants with indications of drug use in 1988, increasing from 9,202 in 1986 (U.S. General Accounting Office [GAO], 1990a). Because of lack of rigorous nationwide detection procedures, and the wide variance in estimates of incidence among hospitals, those numbers are considered a significant underestimation. The 1989 National Drug Control Strategy report estimated that, of the 3.5 million to 4 million annual live births, there are at least 100,000 infants who are affected by their mothers’ cocaine use during pregnancy (U.S. GAO, 1990a).
Maternal cocaine use during pregnancy can impact on child health and behavior in numerous ways. First, maternal cocaine use during pregnancy has the potential to directly and permanently damage the developing central nervous system of the fetus, resulting in later behavioral and learning disabilities. Second, associated health and life-style characteristics of the cocaine-using mother, such as poor nutrition, polydrug use, and inadequate prenatal care, also negatively affect fetal development (). Third, chronic maternal cocaine use can alter the caregiving environment of the infant, since it affects maternal psychological status and behavior, and thus can have long-term effects on child cognitive and emotional development (). Fourth, by adversely affecting pregnancy, infants born to cocaine-using mothers may undergo fetal distress, asphyxia, or be born with low birth weight, and/or prematurity (; Zuckerman, Frank, et al., 1989). These medical problems have significant consequences for neurobehavioral outcome independent of exposure to cocaine.
Because maternal cocaine use is most often identified by health practitioners late in the pregnancy, or at the infant’s birth, pediatricians and pediatric psychologists are on the front lines of dealing with the neurodevelopmental consequences of the cocaine-exposed infant. Pediatric psychologists need to be informed about the research and clinical literature that reports on the impact of maternal cocaine use on child health and behavior, as well as the confounding medical and social problems of these families. They will encounter increasing numbers of affected children in pediatric clinics, hospitals, and school settings in the coming decade. Psychologists will also be involved in designing intervention and research programs for these children. This paper reviews the literature on maternal cocaine use and subsequent child medical and psychological health, focusing on the following general areas: (a) pharmacologic and medical effects of cocaine; (b) epidemiologic studies; (c) behavioral and developmental outcome of cocaine-exposed infants; (d) research methodology; and (e) clinical interventions.
COCAINE—PHARMACOLOGIC AND MEDICAL EFFECTS
In terms of fetal effects, animal studies indicate that cocaine readily crosses the placenta and enters the fetal circulation. Cocaine also induces constriction of uterine blood vessels, producing acidosis and possible asphyxia which is further complicated by contraction of the uterus (Woods, Plessinger, & Clark, 1987). This reduction in the supply of oxygen to the fetus has implications for later neurological and cognitive-behavioral development. Abrupt uterine contractions are considered one reason for the higher rate of spontaneous abortions and uterine/placental separation (abruptio placentae) in cocaine-using women (; ). These cocaine-initiated events may cause premature birth and birth asphyxia. Cocaine is also passed readily to the developing infant through maternal breast milk, with at least one report of acute intoxication of an infant through that mechanism (). have outlined five potential mechanisms for cocaine’s effects on infant behavior. Down-regulation of neurotransmitter receptors may result in defective synaptic development. Cerebral hypoxia, as noted above, can relate to deficits in development. Vascular disruption prenatally has been related in some cocaine studies to malformations in offspring. Fetal malnutrition, mediated by prematurity or maternal malnutrition, may result in growth retardation or microcephaly. Specific areas of the fetal brain may be affected by cocaine exposure, instigating alterations in cerebral activity or structure that negatively affect development.
Increased rates of spontaneous abortions, preterm birth, and lowered gestational age have been noted (; ; ; Singer, Song, Warshawsky, & Kliegman, 1991; Zuckerman, Frank, et al., 1989), placing infants at risk for the multiple developmental hazards of prematurity due to immature organ systems. Intrauterine growth retardation, also known to be associated with later child developmental problems, has been found in almost all studies comparing cocaine-exposed to cocaine-free infants (Chasnoff & Griffith, 1990; ; ; Zuckerman, Frank, et al., 1989). Brain growth appears to be similarly affected as determined by small head circumferences. In fact, the most compelling finding to date of the adverse effect of fetal cocaine exposure on infant development is that of intrauterine growth retardation. Several recent studies using adequate sample size, multivariate analyses, careful matching or stratification methods, and control for confounding variables of maternal age, ethnicity, prematurity, and polydrug use, have indicated an independent effect of cocaine on growth retardation (Coles, Platzman, Smith, James, & Falek, 1991; ; ; ; Zuckerman, Frank, et al., 1989).
Most available studies of the development of cocaine-exposed infants have focused on full-term infants. As prematurity and low birth weight appear to be obstetric effects of maternal cocaine use, the follow-up of only full-term cohorts may be misleading and overly optimistic by eliminating the most affected infants. The developmental risks of prematurity may be exacerbated in cocaine-exposed preterms, or alternatively, may be reduced because of lowered fetal exposure. A follow-up study of a cohort of very low birth weight infants with chronic lung disease has indicated that 25% of a cohort born in 1990, in an urban, tertiary-care hospital were cocaine-exposed, more than double the rate of the hospital’s general obstetric sample (Singer, 1990).
Neonatal neurologic abnormalities, including cerebral infarction, EEG, BAER, and ultrasonographic, or pneumographic abnormalities, have been noted in several studies of cocaine-exposed infants (; ; ). These neurological abnormalities may be factors affecting the early developmental problems seen in follow-up studies and have implications for long-term academic and learning disabilities. Iti fitter trade theory pdf free download in hindi. Thus, in utero cocaine exposure has been related to a variety of significant medical and neurologic risks for the infant.
EPIDEMIOLOGIC STUDIES
From 10 to 20% of pregnant women use some illegal drugs during their pregnancies, with a similar incidence found in both minority, low income, and middle class, private care populations (; Hagopian et al., 1990; Zuckerman, Frank, et al., 1989). The most commonly used method of detection of cocaine use in pregnant women in studies to date has been urine screening at delivery. Cocaine’s metabolite, benzoylecgonine, is detectable in urine for 3 to 6 days after use. Thus, studies relying on urine screening alone are likely to underidentify maternal cocaine use; as are studies dependent on only self-report and clinical interview (Zuckerman, Frank, et al., 1989). Newborn meconium can also be screened through radioimmunoassay and is a more sensitive measure of benzoylecgonine than urine assays (). Although not yet well validated, meconium assays, which can potentially identify maternal cocaine use throughout the entire gestation period, are likely to replace urine assays in research programs due to their advantages. Analysis of hair specimens, both in infants and adults, has also been proposed as a potentially accurate and easy method of estimation of duration, degree, and even time of drug exposure (). However, hair analysis remains a controversial method, requiring further study and validation.
Racial and social class differences have been noted as to preferred drugs assessed through anonymous urine toxicology screens. The few available studies suggest that cocaine use is more prevalent among poor minority women and that marijuana use predominates among white, middle-class patients (; Hagopian et al., 1990). The social and legal consequences of detecting drug use during pregnancy may be racially biased. Despite similar rates of illegal drug use detectable in urine screens at their infants’ birth, minority women were found to be ten times more likely to be referred to the Department of Human Services for drug use than white private-care patients ().
Multiple associated social and health risks detrimental to fetal and infant development have been uniformly documented in all studies examining the characteristics of cocaine-using pregnant women. Cocaine-use during pregnancy is associated with heavy and chronic use of additional legal and illegal drugs. Marijuana, alcohol, and cigarette use are increased at almost three times the rate found in non-cocaine-using samples from similar racial and social class groups (; ; Singer, Song, et al., 1991). Other commonly used substances include methadone, heroin, amphetamines, PCP, barbiturates, LSD, and diazepam. These confounding drugs may also adversely effect the outcome of the pregnancy or the neurobehavioral development of the infant. Poor prenatal care is characteristic, with some studies reporting that from 50 to 60% of their sample received no prenatal care at all (; Singer Song, et al., 1991).
Cocaine-using women weigh less at the birth of their infant, and may gain less weight during their pregnancies, suggesting that anorexia and poor maternal nutrition, known side effects of chronic cocaine usage, may exacerbate any direct effect of cocaine on fetal growth (; Singer, Song, et al., 1990). Rates of infection are also high in cocaine-using women, particularly of sexually transmitted diseases, and in some areas of the country, of HIV and syphilis, both of which adversely affect perinatal outcome. Cocaine-using women report that their infants’ fathers also use cocaine and other drugs (). Paternal use of drugs is an important, as yet unquantified, issue in assessing the sequelae of cocaine exposure in infants, since animal studies have implicated paternal use as a significant factor in evaluating the effects of other teratogens on offspring development. Variations in prenatal timing, dosage, frequency, and patterns of cocaine and other drug use among pregnant women which may affect perinatal and infant outcome may be significant and need to be studied. Social class, cultural attitudes, and regional differences in drug availability, purity, potency and mode of use, and associated nutritional, medical care, and life-style differences also await further epidemiologic study as these factors may worsen or mitigate cocaine’s effects on fetal development.
BEHAVIORAL AND DEVELOPMENTAL OUTCOMES
The general public has been familiarized with child developmental problems associated with the cocaine epidemic through the popular media. Alarming but unsystematic reports on “crack babies” and “coke kids” have pervaded the public consciousness. These reports have characterized cocaine-exposed children in such terms as “joyless, inconsolable, unmotivated, unable to learn, incapable of normal human attachment, aggressive, and hyperactive” (Blakeslee, 1990; Chira, 1990). Currently, there are no available studies of the development or behavior of cocaine-exposed infants beyond 3 years of age. Thus, no firm conclusions can be drawn regarding the possible severity of, or types of, abnormal developmental outcomes associated with fetal cocaine exposure. These dramatic clinical examples may be the exception rather than the rule. Such severe patients may be highlighted by the media but may not be representative of the characteristics of the entire population of cocaine-exposed infants.
The clinical literature does have serious concerns about the social behaviors and educational needs of cocaine-exposed children. In areas of the country where the crack-cocaine epidemic has become entrenched, child welfare departments have documented significant increases in the need for infant and child foster care. Although it is generally accepted that parental abandonment, abuse, and neglect of children are associated with increases in maternal drug use (; U.S. GAO, 1990a), there is variation among states on current legislative practices in child abuse and neglect (Marshall, 1991). The recent explosive increase in maternal drug use has prompted innovative alternatives for protective custody including in-home intensive programming, home visiting, and residential drug treatment for mothers and children (U.S. GAO, 1990b; Macro Systems, Inc., 1991; LRP, 1991). Many of these programs have begun only recently so information about outcomes is not complete.
In addition, an emerging infant developmental literature has begun to document specific neurobehavioral effects of fetal cocaine exposure on maternal and infant behavior. These studies have focused on infant behavior, infant cognitive development, and maternal–child interaction.
NEONATAL BEHAVIOR
In their pioneering studies, Chasnoff and colleagues described cocaine-exposed neonates as different from non-drug-exposed infants (; ). Lethargy, poor responsivity, irritability, tremulousness, hypertonicity, and disorganization of feeding and sleeping patterns were characteristics described by workers caring for these infants in neonatal nurseries. Subsequently, several standardized scales have been used to describe objectively and to quantify these behavioral characteristics in the fetal and neonatal periods.
To determine whether or not cocaine-exposed infants experienced a withdrawal syndrome-after birth similar to that seen in heroin- or methadone-addicted infants, some investigators have used the Neonatal Abstinence Scale (Finnegan, 1984) with varying results. This scale measures 21 signs of drug withdrawal, including tone, sleeping and feeding irregularities, tremulousness, and tachypnea. An infant showing signs of withdrawal, for example, may cry incessantly, as well as sleep or eat poorly. While some studies (; ) have noted mild withdrawal symptoms in cocaine infants, other reports found no withdrawal symptoms in cocaine infants in controlled studies (Coles et al., 1991; Finnegan, Kaltenbach, Weiner, & Haney, 1990). Since withdrawal scales designed for narcotic drugs may not be relevant to cocaine, other measures may be more appropriate for describing the spectrum of behaviors seen in cocaine-exposed neonates.
All but one study (Woods, Eyler, Behnke, & Conlon, in press) have found sensory and behavioral deficits in cocaine-exposed neonates in comparison to social class and racially matched controls using the Neonatal Behavioral Assessment Scale (NBAS, Brazelton, 1973). The NBAS was developed to assess the behavioral repertoire of the full-term infant from birth through the first month of life. Items assess visual and auditory orientation, habituation, reflex behavior, physiological stability, and interactive behavior with the examiner, including ability to alert and attend. These items are typically expressed through cluster scores. Abnormalities in comparison to non-cocaine-exposed infants have been found across several studies which examined cocaine-exposed infants with the NBAS in the neonatal and early infant periods (; Coles et al., 1991; ; ).
Several of these studies have had adequate sample sizes so that some control could be made for potentially confounding variables. assessed 26 primarily black, lower social class, full-term cocaine-exposed neonates within 1 week of urine toxicology screen and, at a standardized test time (i.e., approximately 1 hour after feeding). Control infants were matched based on a random stratification procedure on sex, ethnicity, gestational age, and birth weight. Groups were equivalent on maternal age, gravidity, abortion history, and presence of hepatitis. While mothers who used opiates were excluded, cocaine-using women were found to have higher use of cigarettes and alcohol. Marijuana use was reported by 39% of the cocaine group, but was an exclusionary factor for controls. Depressed habituation scores on the NBAS were noted in cocaine-exposed newborns, indicating that cocaine-exposed neonates were less adept at screening out aversive stimulation than comparison infants. Results from a stepwise regression analysis indicated maternal cocaine use to be the only significant variable entering the equation, predicting a significant amount of variance in infant habituation score. Since habituation is an early form of learning, which allows the infant to screen out aversive or redundant stimuli, decreased habituation skills may be a factor in the cocaine-exposed infant’s reported irritability and inconsolable behaviors.
A longitudinal investigation at 2, 14, and 28 days postnatal age was undertaken by Coles et al. (1991), also using only full-term, healthy infants. This study controlled experimentally for gestational age, other drug use, associated maternal illness, and duration of drug use. Maternal drug use was assessed via self-report and urine screening. Infants of women who used cocaine or alcohol, and no other drugs except cigarettes and marijuana, were recruited. Comparison infants were mothers of similar age, race, and social class. Full-term healthy infants were examined at 2, 14, and 28 days, and grouped as follows: no cocaine or other drugs, cocaine-positive postpartum, cocaine-negative postpartum, and alcohol only. Behavioral outcome was measured through NBAS subscales, except for habituation, for which there were inadequate data. While no group differences were found at 2 days, cocaine-exposed infants who were positive postpartum showed poorer autonomic regulation at 14 and 30 days, and had more abnormal reflexes at 30 days than the no-cocaine group. Using a simultaneous regression model to assess independent and interactive effects of cocaine with other drugs on infant behavioral outcome, this study found duration and/or frequency of cocaine use to contribute significant variance in NBAS cluster scores at all ages. The effects were most pronounced by 28 days, when cocaine independently predicted abnormalities in motor and reflex behavior and state regulation. The importance of assessment of polydrug use was also demonstrated in this study, as marijuana, alcohol, and cigarette use, either alone or in interaction with cocaine, also predicted NBAS differences.
Less optimal infant behavior on NBAS assessment was noted in another study which followed term, cocaine-exposed and non-exposed infants at birth and at 1 month of age (). No differences were found on NBAS clusters at birth; however, cocaine-exposed infants demonstrated reliably poorer motor responses at follow-up. Cocaine exposure accounted for a significant portion of the motor cluster score variance, but not when potentially confounding variables, including other drugs, perinatal variables, and demographic and examination factors, were controlled. The high rate of attrition (60%) in this study limits these findings, particularly as sample size in the cocaine group for the regression analysis was inadequate for the large number of independent variables considered.
Using a matched design to control for confounding variables of race, parity, tobacco use, intrauterine growth, and delivery type, Woods et al., in press) assessed cocaine-exposed and non-exposed infants on the NBAS immediately after birth and at 1 month. They found no differences between groups; at either time. However, as infants were assessed at less than 3 days of age, the effects of other medications used in delivery for both groups may have: obscured differences. As with Coles’s study, sample size for the habituation cluster was much smaller than the overall sample size. At 1 month follow-up, sample size was reduced such that power to detect differences may have been inadequate.
Thus, of the few currently available studies, there have been no consistent findings related to specific newborn behavioral abnormalities in term, healthy cocaine-exposed infants, perhaps due to methodologic differences in sample selection, variation in control for various confounds, timing and nature of assessment instruments, and attrition rates. Only one study (Coles et al., 1991) had adequate sample size beyond the immediate newborn period to assess the effects of cocaine, relative to other drugs and related to duration and frequency of use, on infant behavior. This study suggests both independent and interactive effects of cocaine on infant behavior that warrant further study.
COGNITIVE DEVELOPMENT
Beyond the newborn period, cocaine-exposed infants have been reported to score similarly to non-cocaine-exposed infants on standard IQ tests through 3 years of age, the length of time on which some follow-up is available (Griffith, Chasnoff, Gillogley, & Frier, 1990). Only children of mothers who had entered a prenatal drug treatment program and who may not be representative of general sample of cocaine-exposed infants were studied, however. The level of play behavior, also a correlate of cognitive development, may also be affected. Using play behavior, one study (Rodning, Beckwith, & Howard, 1989a) found preterm toddlers exposed to cocaine and other drugs to show poorly developed play behaviors, and a lack of interest and motivation in unstructured situations, in comparison to a group of high risk preterm children. Although this study was inconclusive due to methodologic problems, the use of a standardized free play assessment during which levels of manipulative, functional, and symbolic play were rated may be useful in providing information about cognitive processing deficits not apparent in typical IQ testing.
Although current findings are preliminary, they are consistent with clinical and research reports of early behavioral abnormalities in the neonatal period, and with human and animal studies that document fetal problems of hypoxia and growth retardation known to be related to neurological development and functioning. Thus, cocaine-exposed infants appear to be at risk for later learning and behavioral disabilities from a biological perspective.
CARETAKING AND MATERNAL–INFANT INTERACTION
As environmental and caretaking factors also exert considerable effects on the long-term outcome of children, these factors must be considered in evaluating risk in cocaine-exposed infants. In a 10-hospital national study, the U.S. General Accounting Office (1990a) found that approximately 1,200 of the 4,000 drug-exposed infants born in 1989 surveyed were placed in foster care, suggesting a serious level of neglect and abuse requiring out-of-home placement. While foster placement may be considered a better alternative than the often chaotic and dangerous environment associated with maternal drug use, the poor quality of many foster homes and the lack of supportive health and social services to foster care families also may be detrimental. Because the estimated demand for foster care has increased nationwide by 29% from 1986 to 1989, it is questionable whether an already beleaguered social welfare system can adequately meet the increased needs of drug-exposed children (U.S. GAO, 1990a).
For those infants who return home from the hospital with their mothers, the effects of maternal drug life-style must be considered. For example, the impact of an impoverished home environment in which available resources are funneled into drug procurement can only be negative. Domestic violence, child neglect and abuse, and poor health care directly and negatively affect child development. The drug life-style may also have direct toxic effects on neurological development as infants and children passively inhale smoke in the crack-cocaine user’s environment (). wrote that the overlap of drug use and social problems for this target population warrant comprehensive monitoring of maternal depression and confusion, as well as interventions aimed at the restoration of self-esteem, promotion of better parenting skills, and addressing episodes of spouse abuse as an alternative to a sole referral to child welfare services.
Little is known about the interactional and caretaking behaviors of cocaine-using women. reviewed the effects of womens’ alcohol and drug use on parenting skills. Women who abuse drugs often have negative self-concepts and poor tolerance for frustration which leads to ineffective parenting and ambivalence about motherhood. Maternal psychological status and interaction with her infant may be impaired through drug intoxication or withdrawal (Chethick, Burns, Burns, & Clark, 1990). Several preliminary studies are examining the caretaking interactions of cocaine-using mothers with conflicting findings (Fitzgerald, Kaltenbach, & Finnegan, 1990; ). Fathers of drug-exposed infants, 50% of whom are reported to also use drugs (), also have not been studied. Some reports suggest they are often involved in a criminal or violent life-style which would impact on child emotional and behavioral outcomes (Howard, Beckwith, Rodning, & Kropenske, 1989).
Maternal depressive symptoms, either as a precipitating factor or as a consequence of cocaine use or drug life-style, may also mediate infant developmental outcome in cocaine-exposed populations. Cocaine use has been linked to depressive symptoms in pregnant women (Zuckerman, Amaro, Bauchner, & Cabral, 1989), and depression has been noted as a correlate of withdrawal or “crash” stages of chronic cocaine use (). Infants of depressed mothers have been considered at risk for later emotional problems due to alterations in maternal and infant behaviors shown in infants of healthy mothers simulating depression (), and postpartum depressed mothers (Cohn, Campbell, Matias, & Hopkins, 1990; Field, 1984). Assessment of depressive, as well as other psychological symptoms that might affect maternal behavior and care of her infant appear to be an important research focus.
Using the short version of the Beck Depression Inventory, one study (Woods et al., in press) compared cocaine-using women to non-cocaine-using women immediately postpartum and 1 month later. Higher levels of depressive symptomatology were reported immediately after birth in the cocaine group, but were diminished by 1 month. However, significant sample attrition (45%) by the follow-up assessment attenuated the validity of these findings. Further studies of the depressive and/or other psychological symptoms of cocaine-using mothers are needed to provide greater information about maternal personality and emotional status and their impact on child outcome.
One aspect of early infant development thought to be a correlate of later emotional competence has been the consolidation of a secure attachment to a primary caregiver in the first year of life. Through the experimental “strange situation” paradigm (Ainsworth, Blehar, Waters, & Walls, 1978), secure and insecure attachments have been examined in normal and at-risk populations, including failure-to-thrive (Valenzuela, 1990), chronically ill (), and abused infants (). Using this paradigm, one study () found drug-exposed toddlers, some of whom had been exposed to cocaine in utero, to be at greater risk for insecure and anxious caregiver attachments. Although this study was limited by a small sample and lack of control for many confounding factors, such as foster care, multiple drug use, and prematurity, the issue of attachment in cocaine-exposed infants warrants further investigation as an early indication of potential developmental and emotional problems.
In summary, in addition to constitutional factors, which place the cocaine-exposed infant at high risk for long-term developmental problems, chronic poverty, altered caregiver interaction, modeling of a drug life-style, and the high potential for exposure to violence, abuse, and neglect sustain and exacerbate the developmental risk to these infants.
RESEARCH METHODOLOGY
Research on the prevalence of maternal cocaine use during pregnancy and subsequent developmental sequelae for the cocaine-exposed infant can be strengthened by addressing the possible sources of bias in current research, which include issues of sampling, choice of measures, timing of measures, and response rates. To date, the majority of epidemiologic studies have focused on low-income, minority urban women. Studies that use randomized or stratified random techniques to select a sample from a frame that includes all income groups, as well as urban and rural settings, will increase knowledge about middle-class white or rural women and how their health care, obstetric risk, and later caregiving behaviors are affected by cocaine use during pregnancy. Obtaining accurate information about cocaine use during pregnancy is problematic. Many drug users tend to deny their addictions; additionally, the detection of use of an illegal drug may impose penalties on the pregnant woman, including incarceration and referral of the infant to child welfare services (English, 1990). Policies that safeguard the respondent’s confidentiality and a clear protocol for explaining those safeguards must be part of the design of studies to minimize refusals and/or a tendency to underreport use because of fear. Cocaine use is not routinely assessed in prenatal care, and may be missed in women who do not seek prenatal care. Routinely, including multiple indicators (e.g., urine toxicology screens and/or meconium screening, and questions about cocaine and other drug use) for all obstetrical patients regardless of income level, and regardless of the amount of prenatal care received will aid in understanding the differences and similarities of drug use among various racial groups and social classes.
As cocaine-using women also show increased use of other legal and illegal drugs also known to affect infant outcome, the impact of other substances should be considered in studies of cocaine exposure. Urine toxicology screens can identify only relatively recent users of the drug, leaving women whose use may have been confined to earlier trimesters unnoticed. Some studies have enrolled only women engaged in addiction treatment programs prenatally. However, only approximately 50% of cocaine-using women enter drug treatment programs during pregnancy and up to 60% of cocaine-using women may receive no prenatal care at all (Singer, Song, et al., 1991). Follow-up studies should include children whose mothers were not in treatment prenatally, as well as those whose mothers did receive treatment to capture the spectrum of effects produced by maternal cocaine use and to increase generalizability to the majority of cocaine-exposed infants. Studies of preterm cocaine-exposed infants are needed as well, to follow-up on the fact that prematurity has been frequently noted as one of the sequelae of maternal cocaine usage during pregnancy.
Research into the effects of fetal cocaine exposure is relatively new. Building upon the information initially gleaned from studies with small samples, future researchers should obtain samples that minimize the bias tied to race and social class, and should employ designs that include appropriate comparison groups. Follow-up studies should extend through the school age years when learning and behavioral deficits usually become evident, and should incorporate instruments that have adequate standardization as well as known reliability. The numerous confounding factors related to maternal cocaine use (i.e., poor maternal nutrition and lack of prenatal care, polydrug use, infections, and poverty) make it difficult to disentangle the effects of fetal cocaine exposure per se from the effects of other deleterious obstetric and environmental factors on child development and behavior. However, initial studies documenting intrauterine growth retardation, and the known independent negative effects of these confounding factors, suggests significant clinical risk to the cocaine-exposed infant ().
CLINICAL INTERVENTIONS
The expertise of pediatric psychologists is needed to design and provide clinical services to the growing number of infants and children exposed to crack-cocaine in utero. One necessary component to the provision of appropriate interventions is an understanding of the neurobehavioral and social-emotional effects of fetal drug exposure. This data base is currently being hastily compiled because of the pressing need for accurate information regarding the development of drug-exposed infants and children. A second critical component involves educating pediatric psychologists about the special needs of the cocaine-using parent.
Even though the literature pertaining to cocaine use among pregnant women and mothers is only now emerging, there is a relatively large body of literature on the impact and treatment of drug use among women that can be useful in the development of treatment plans and service delivery systems for women who abuse cocaine (; Gomberg, 1986; ; Mondanaro, 1989; Reed, Beschner, & Mondanaro, 1982; Tamerin, 1987).
Tittle and St. Claire (1989) have outlined the specialized needs of the crack-cocaine-using mother, her infant, and the dyad. Special needs of the mother include drug treatment, emotional support, and help in securing basic needs. Special infant needs may include prematurity, possible withdrawal symptoms, and neurobehavioral problems. The pediatric psychologist must also be aware of and assess potential neurobehavioral deficits of the crack-exposed infant which may make parenting difficult. This high risk maternal-infant dyad may be in need of social supports, organization, and in some cases, intensive infant-parent psychotherapy. Some programs have developed special protocols for positioning, carrying, and handling cocaine-exposed infants. Techniques such as swaddling and hydrotherapy to calm the baby, and structured activities and education to facilitate maternal–infant attachment have been recommended (Schneider & Chasnoff, 1987). Cocaine-exposed infants have been characterized as both “overstimulated” and in need of calming, and as “underaroused” and in need of external sources of motivation. Some support for both characteristics was found in a study () that found both excitable and depressed neurobehavioral syndromes in cocaine-exposed newborns.
Other programs have used a case-management approach offering medical and support services as well as early identification of infant developmental delays. Within a program context of a nonjudgmental, caring environment, practitioners have noted improvement in drug-using mothers’ confidence and ability to parent (Tittle & St. Claire, 1989). The pediatric psychologist working in settings with cocaine-exposed infants needs specialized knowledge related to crisis intervention for failure-to-thrive, child neglect and abuse, maternal dual-diagnosis, AIDS, and domestic violence, all of which are more likely to occur in families in which parents use drugs (). Basic health care, safety, housing, and nutritional needs of the infants are threatened by living with a caretaker preoccupied with drug procurement.
Preliminary clinical reports of toddlers and older preschoolers have suggested that cocaine-exposed children exhibit patterns of behavioral problems similar to those seen in emotionally disturbed children. Poor motivation to learn, difficulty organizing responses, sudden mood swings, and slow language acquisition are symptoms noted in crack-exposed children which require special educational efforts (Blakeslee, 1990; Chira, 1990). Pilot programs have used intensive one-to-one teaching and environmental structure along with multimodal cues to enhance learning, emotional control, and organization in cocaine-exposed children (Blakeslee, 1990; Chira, 1990). It remains an open question as to how effective these programs will prove, and to what extent in utero cocaine exposure will result in significant later behavioral and learning disabilities. There is no descriptive data base on school-age or older preschool children exposed to crack-cocaine prenatally, partly because identified cohorts of children are only now reaching school age. Of note is the fact that many descriptions of cocaine-exposed toddlers and preschoolers are similar to those of other at-risk children. As yet, no specific crack-cocaine behavioral syndrome has been identified, and current intervention available to treat other high-risk children may prove fruitful for drug-exposed children.
SUMMARY
Available studies suggest that prenatal exposure to crack-cocaine can have significant medical effects on the fetal growth and neurological development of the infant, potentially resulting in long-term behavioral and intellectual disabilities. Maternal and environmental correlates of crack-cocaine use, such as poverty, domestic violence, foster care, and neglect, also negatively affect cognitive and emotional competence in the over 100,000 infants born annually with exposure to cocaine. Large, population-based studies using multivariate analyses are necessary to determine the independent effects of cocaine on child developmental outcome relative to confounding medical and environmental variables. Pediatric psychologists are in a position to take leadership roles in designing and participating in research and intervention programs to address the negative consequences of fetal cocaine exposure in this growing, at-risk group of children.
Footnotes
1We thank Rose Marie Ashley, Vicki Pozzuto, and Sonia Minnes for help with manuscript preparation. Writing of this paper was supported, in part, by grants from the Rainbow Board of Trustees, the George Gund Foundation, the Cleveland Foundation, the March of Dimes Foundation (12-275), and grants NIH R01HL 38193 and MCJ 390592.
Contributor Information
Lynn Singer, Case Western Reserve University School of Medicine.
Kathleen Farkas, Case Western Reserve University.
Robert Kliegman, Case Western Reserve University School of Medicine.
REFERENCES
- Ainsworth M, Blehar M, Waters E, Walls S. Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Bateman DA, Heagarty MC. Passive free base cocaine (‘crack’) inhalation by infants and toddlers. American Journal of Diseases in Childhood. 1989;143:25–27. [PubMed] [Google Scholar]
- Bays J. Substance abuse and child abuse. Pediatric Clinics of North America. 1990;37:881–903. [PubMed] [Google Scholar]
- Beckman LJ. Women alcoholics: A review of social and psychological studies. Journal of Studies on Alcohol. 1975;36:797–834. [PubMed] [Google Scholar]
- Blakeslee S. Crack’s toll among babies. New York Times. 1990 Sep 17;:1–12.[Google Scholar]
- Brazelton TB. Neonatal Behavioral Assessment Scale. Philadelphia, PA: Spastics International; 1973. [Google Scholar]
- Chasnoff IJ, Burns WJ, Schnoll SH, Burns K. Cocaine use in pregnancy. New England Journal of Medicine. 1985;313:666–669. [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith D, MacGregor S, Dirkies K, Burns K. Temporal patterns of cocaine use in pregnancy. Journal of The American Medical Association. 1989;261:1741–1744. [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith D. Cocaine-exposed infants: Two year follow-up. Pediatric Research. 1990;25:249A.[Google Scholar]
- Chasnoff IJ, Hevet CE, Kletter R, Kaplan D. Perinatal cocaine exposure is associated with respiratory pattern abnormalities. American Journal of Diseases of Childhood. 1989;143:583–587. [PubMed] [Google Scholar]
- Chasnoff IJ, Landress H, Barrett M. The prevalence of illicit drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas County, Florida. New England Journal of Medicine. 1990;322:1202–1206. [PubMed] [Google Scholar]
- Chasnoff I, Lewis DE, Squires L. Cocaine intoxication in a breast-fed infant. Pediatrics. 1987;80:836–838. [PubMed] [Google Scholar]
- Cherukiri R, Minkoff J, Feldman J, Parekh A, Glass L. A cohort study of alkaloidal cocaine (“crack”) in pregnancy. American Journal of Obstetrics and Gynecology. 1988;157:686–690. [PubMed] [Google Scholar]
- Chethick L, Burns K, Burns W, Clark R. The assessment of early relationship dysfunction in cocaine-abusing mothers and their infants. Infant Behavior and Development. 1990;13:312.[Google Scholar]
- Chira S. Crack babies turn five, and schools brace. New York Times. 1990 May 25;:A1–A11.[Google Scholar]
- Cohn J, Tronick E. Three month old infants’ reactions to simulated maternal depression. Child Development. 1983;54:186–193. [PubMed] [Google Scholar]
- Cohn J, Campbell S, Matias R, Hopkins J. Face-to-face interactions of post-partum depressed and non-depressed mother-infant pairs at two months. Developmental Psychology. 1990;22:15–22.[Google Scholar]
- Coles CD, Platzman K, Smith I, James M, Falek A. Effects of cocaine, alcohol, and other drugs used in pregnancy on neonatal growth and neurobehavioral status. Neurotoxicology and Teratology. 1991;13:1–11.[Google Scholar]
- Cregler LL, Mark H. Medical complications of cocaine abuse. New England Journal of Medicine. 1986;315:1495–1500. [PubMed] [Google Scholar]
- Crittenden P. Maltreated infants: Vulnerability and resilience. Journal of Child Psychology and Psychiatry. 1985;26:85–96. [PubMed] [Google Scholar]
- Davis SK. Chemical dependency in women: A description of its effects and outcome on adequate parenting. Journal of Substance Abuse Treatment. 1990;7:225–232. [PubMed] [Google Scholar]
- Dixon S. Effects of transplacental exposure to cocaine and methamphetamine on the neonate. Western Journal of Medicine. 1989;150:436–442.[PMC free article] [PubMed] [Google Scholar]
- Dixon S, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: Incidence and correlates. Journal of Pediatrics. 1989;117:770–778. [PubMed] [Google Scholar]
- Doberczak T, Shanzer S, Senie R, Kandall S. Neonatal neurologic and electroencephalographic effects of intrauterine cocaine exposure. Journal of Pediatrics. 1989;117:354–358. [PubMed] [Google Scholar]
- Eisen L, Field T, Bandstra E, Roberts J, Morrow C, Larson S, Steele B. Perinatal cocaine effects on neonatal stress behavior and performance on the Brazelton Scale. Pediatrics. 1991;88:477–480. [PubMed] [Google Scholar]
- English A. Prenatal drug exposure: Grounds for mandatory child abuse reports? Youth Law News. 1990;11:3–8.[Google Scholar]
- Field T. Early interactions between infants and their post-partum depressed mothers. Infant Behavior and Developments. 1984;7:527–532.[Google Scholar]
- Finnegan LP. Neonatal abstinence. In: Nelson M, editor. Current therapy in neonatal and perinatal medicine. City, Ontario, Canada: B. C. Decker; 1984. [Google Scholar]
- Finnegan L, Chappel J, Kreek MJ. Narcotic addiction in pregnancy. In: Niebyl JR, editor. Drug use in pregnancy. Philadelphia, PA: Lea & Febiger; 1982. [Google Scholar]
- Finnegan L, Kaltenbach K, Weiner S, Haney B. Neonatal cocaine exposure: Assessment of risk scale. Pediatric Research. 1990;25:10A.[Google Scholar]
- Fisher-Fay A, Goldberg S, Simmons RJ. Chronic illness and infant-mother attachment. Journal of Developmental and Behavioral Pediatrics. 1988;9:266–270. [PubMed] [Google Scholar]
- Fitzgerald E, Kaltenbach K, Finnegan L. Patterns of interactions among drug dependent women and their infants. Pediatric Research. 1990;25:10A.[Google Scholar]
- Frank D, Zuckerman BS, Amaro H, Aboagye K, Bauchner H, Cabral H, Fried L, Hingson R, Kayne H, Levenson S, Parker S, Reece H, Vinci R. Cocaine use during pregnancy: Prevalence and correlates. Pediatrics. 1988;82:888–895. [PubMed] [Google Scholar]
- Frank D, Bauchner H, Parker S, Huber A, Kejer-Aboagye K, Cabral H, Zuckerman B. Neonatal body proportionality and body composition after in utero exposure to cocaine and marijuana. Journal of Pediatrics. 1990;117:622–626. [PubMed] [Google Scholar]
- Gawin F. Chronic neuropharmacology of cocaine: Progress in pharmacotherapy. Journal of Clinical Psychiatry. 1988;49:11–16. [PubMed] [Google Scholar]
- Gillogley K, Evans A, Hansen R, Samuels S, Batra K. The perinatal impact of cocaine, amphetamine, and opiate use detected by universal intrapartum screening. American Journal of Obstetrics and Gynecology. 1990;163:1535–1542. [PubMed] [Google Scholar]
- Gomberg ESL. Women with alcohol problems. In: Estes N, Heinemann M, editors. Alcoholism: Development, consequences, and interventions. 3rd ed. St. Louis, MO: C. V. Mosby; 1986. pp. 241–256. [Google Scholar]
- Graham K, Korero G, Klein J. Determination of gestational cocaine exposure by hair analysis. Journal of the American Medical Association. 1989;262:3328–3330. [PubMed] [Google Scholar]
- Griffith D, Chasnoff IJ, Gillogley K, Frier C. Developmental follow-up of cocaine-exposed infants through age three years. Infant Behavior and Development. 1990;13:126A.[Google Scholar]
- Hadeed A, Siegel S. Maternal cocaine use during pregnancy: Effect on the newborn infant. Pediatrics. 1989;84:205–210. [PubMed] [Google Scholar]
- Hagopian E, Darby M, Patti L, Knee G, Kaltenbach K, Finnegan L. The Prevalence of antepartum substance abuse. Pediatric Research. 1990;25:207A.[Google Scholar]
- Howard J, Beckwith L, Rodning C, Kropenske V. The Development of young children of substance-abusing parents. Zero to Three. 1989;9:8–12.[Google Scholar]
- Lester B, Als H, Brazelton TB. Regional obstetric anesthesia and newborn behavior. Child Development. 1982;53:687–692. [PubMed] [Google Scholar]
- Lester B, Corwin M, Sepkoski C, Seiper R, Peucker M, McLaughlin S, Golub H. Neurobehavioral syndromes in cocaine-exposed newborns. Child Development. 1991;62:694–705. [PubMed] [Google Scholar]
- Lief N. The drug user as parent. International Journal of the Additions. 1985;20:63–97. [PubMed] [Google Scholar]
- L. R. P. Publications. Babies and cocaine: Children and families at-risk. Alexandria, VA: Author; 1991. [Google Scholar]
- MacGregor S, Keith L, Bachicha J, Chasnoff IJ. cocaine use during pregnancy: Correlation between prenatal care and perinatal outcome. Obstetrics and Gynecology. 1989;74:882–885. [PubMed] [Google Scholar]
- Macro Systems, Inc. Programs serving drug-exposed children and their families. Vol. I: Cross-site findings and policy issues. Washington, DC: Submitted to Assistant Secretary for Planning & Evaluation U.S. Dept. of Health & Human Services; 1991. [Google Scholar]
- Marshall AB. Perinatal Addiction Research and Education Update. Chicago, IL: NAPARE; 1991. Sep, State-by-state legislative review; p. 1. [Google Scholar]
- Mondanaro J. Chemically dependent women: Assessment and treatment. Lexington, MA: Lexington Books; 1989. [Google Scholar]
- Neuspiel D, Hamel S. Cocaine and infant behavior. Developmental and Behavioral Pediatrics. 1991;12:55–64. [PubMed] [Google Scholar]
- Neuspiel D, Hamel S, Hochberg E, Green J, Campbell D. Maternal cocaine use and infant behavior. Neurotoxicology and Teratology. 1991;13:229–233. [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: Maternal and neonatal correlates. Journal of Pediatrics. 1987;111:571–578. [PubMed] [Google Scholar]
- Ostrea EM, Brady M, Parks P, Asensio D, Naluz A. Drug screening of meconium in infants of drug-dependent mothers: An alternative to urine testing. Journal of Pediatrics. 1989;115:474–477. [PubMed] [Google Scholar]
- Reed BG, Beschner GM, Mondanaro J, editors. Treatment services for drug dependent women. Vol. 2. Rockville, MD: National Institute on Drug Abuse; 1982. [Google Scholar]
- Regan DA, Ehrlich SM, Finnegan LP. Infants of drug addicts: At-risk for child abuse, neglect, and placement in foster care. Neurotoxicology and Teratology. 1987;9:315–319. [PubMed] [Google Scholar]
- Rodning C, Beckwith L, Howard J. Characteristics of attachment organization and play organization in prenatally drug-exposed toddlers. Developmental Psychopathology. 1989a;1:277–289.[Google Scholar]
- Rodning C, Beckwith L, Howard J. Prenatal exposure to drugs: Behavioral distortions reflecting CNS impairment? Neurotoxicology. 1989b;10:629–634. [PubMed] [Google Scholar]
- Schneider J, Chasnoff IJ. Cocaine abuse during pregnancy: Its effects on infant motor development: A Clinical perspective. Topics in Acute Care and Trauma Rehabilitation. 1987;2(1)[Google Scholar]
- Singer LT. Follow-up of very low birth weight infants with chronic lung disease. 1990. Unpublished raw data. [Google Scholar]
- Singer LT, Garber R, Kliegman R. Neurobehavioral sequelae of fetal cocaine exposure. Journal of Pediatrics. 1991;119:667–672.[PMC free article] [PubMed] [Google Scholar]
- Singer LT, Song L, Warshawsky E, Kliegman R. Maternal gravidity predicts prematurity in cocaine-exposed infants. Pediatric Research. 1991;26:266A.[Google Scholar]
- Tamerin J. The psychotherapy of alcoholic women. In: Zimberg S, Wallace J, Blum S, editors. Practical approaches to alcoholism psychotherapy. 2nd ed. New York: Plenum Press; 1987. pp. 259–278. [Google Scholar]
- Tittle B, St. Claire N. Promoting the healthy development of drug-exposed children through a comprehensive clinic model. Zero to Three. 1989;9:18–19.[Google Scholar]
Cocaine abuse and addiction continue to be a problem that plagues the United States. Cocaine is a highly addictive drug, currently a Schedule II substance. Cocaine is categorized as a stimulant.
Like most stimulants, cocaine can heighten activity in the body, including heart rate, blood pressure, alertness, and energy. The most commonly used form of the drug is a white powder which is found in the leaves of the Erythroxylon Coca plant, which has been used in South America for hundreds of years.
First introduced in the United States in the 1880s as a surgical anesthetic, cocaine soon began being used as a common household drug, as well as an ingredient in Coca-Cola and other drinks. It was classified as a Schedule II drug in 1970.
What Is Cocaine?
Cocaine is one of the oldest known psychoactive substances. The leaves of the Erythroxylon coca bush have been chewed and ingested for thousands of years. Cocaine hydrochloride, the purified chemical extracted from the plant has been abused for more than 100 years.
In the early 1900s, cocaine was the active ingredient in many of the tonics and elixirs that were marketed at the time to treat a variety of conditions and illnesses. It was an ingredient in the original formula for the soft drink Coca-Cola.
The peak of the drug's popularity came in the 1980s and 1990s when it was known by names like the Movie Star Drug and California Cornflakes.
Cocaine is a very addictive stimulant that directly affects the brain. It is a Schedule II drug which has the high potential for abuse, but can also be administered for legitimate medical purposes, such as a local anesthetic.
However, cocaine is mostly sold on the street illegally as a fine white powder. Many times it is mixed with other substances like cornstarch, talcum powder or sugar to dilute its purity. Sometimes it is mixed with amphetamine or with heroin in what is known as a 'speedball.'
Cocaine is also sold on the street in a freebase form known as crack cocaine. The base form of cocaine is processed with ammonia or baking soda and water then heated to remove the hydrochloride to produce a smokable version of the drug.
The term 'crack' refers to the crackling sound the substance makes when it is smoked.
What Is Crack Cocaine?
When powdered cocaine hydrochloride is processed into a smokable substance it is called freebase, or in street terms, crack cocaine. The term 'crack' actually refers to the crackling sound the freebase form of the drug makes when it is burning.
Using ammonia or sodium bicarbonate (baking soda) and water, powdered cocaine is heated to remove the hydrochloride. This produces the freebase, or smokable form of the drug.
When users smoke crack cocaine the experience a high almost immediately (usually less than 10 seconds). Because the high is immediate and euphoric and because crack is relatively inexpensive to produce and buy on the street, the drug became extremely popular in the mid-1980s.
The immediate high and the relatively quick 'crash' after the first rush, is also the reason that crack cocaine is very addictive.
What Is the Scope of Cocaine Use in the United States?
The number of current cocaine users has been declining steadily since the 1980s and that decline has continued into the 21st Century. According to the National Survey on Drug Use and Health (NSDUH), in 2012 there were 1.6 million cocaine users aged 12 or older, or about 0.6% of the population.
That number is similar to the 2011 rate (1.4 million and 0.5%) but significantly lower than the current cocaine users between 2003 and 2007 (2.4 million users or 1.0%).
Although the greatest number of cocaine users are young adults between age 18 and 25, from 2005 to 2012 the number of current users in that age bracket dropped from 2.6% to only 1.1%.
Additionally, the number of new cocaine users is also declining. The number of people who initiated cocaine use for the first time during the past year dropped from 1.0 million in 2002 to 639,000 in 2012.
Likewise, the Monitoring the Future survey, which surveys 8th-, 10th- and 12th-grade students annually, has shown a steady decline in past-month cocaine use by students from a peak in the 1990s through 2013.
How Is Cocaine Used?
Cocaine can been taken by various methods: oral, intranasal, intravenous, and inhalation. Or, as these methods are known on the street, 'chewing,' 'snorting,' 'mainlining,' 'injecting,' and 'smoking.'
Except for approved medical use, there is no safe way to use cocaine in any form. All of the following methods of using the drug can lead to absorption of toxic levels of cocaine, possible acute cardiovascular or cerebrovascular emergencies, and seizures, according to the National Institute on Drug Abuse. Any of these can lead to sudden death.
Snorting
Intranasal administration is snorting, the process of inhaling powdered cocaine through the nostrils. It can also be rubbed onto the mucous tissues and absorbed in the bloodstream.
Typically, when a user snorts cocaine, the drug is placed on a flat surface like a mirror and separated into 'lines' with a razor blade or credit card, then the lines are snorted through a straw or a rolled-up dollar bill. In the 1980s it was considered gauche in some circles to snort cocaine with anything but a $100 bill.
Injecting
Intravenous use or injecting is when a hypodermic needle is used to inject cocaine directly into the bloodstream, which increases the intensity of its effects.
Because powder cocaine is actually cocaine hydrochloride, the salt (HCL) makes it soluble in water so that it can be injected. Problems can occur when cocaine purchased on the street is laced with unknown substances that are not so easily soluble.
Smoking
Smoking cocaine involves inhaling smoke or cocaine vapor into the lungs where its absorption into the bloodstream can be almost as rapid as injection. This produces an almost immediate and euphoric effect which is one of the reason smoking crack cocaine became so widespread in the 1980s.
The Method of Use Affects the Effects
When cocaine is snorted, its effects begin after a few minutes and last between 15 to 30 minutes depending on the dosage size and the tolerance of the user. A large dose will last slightly longer, but as the user builds up a tolerance to the drug, it takes larger and larger doses to achieve the same effect.
When cocaine is smoked, the effects of the drug begin almost immediately and intensely, but the effect 'wears off' quickly—in maybe five or 10 minutes. This is one reason that crack cocaine is so addictive, users tend to smoke more and more of it to try to recapture the feeling of that first, intense high.
When cocaine is injected the effect is immediate and even more intense. Because of the intense and quick effect of smoked and injected cocaine, these methods of use are considered more dangerous because of the potential for addiction and the potential of overdose.
How Does Cocaine Produce Its Effects?
Many years of scientific research has been required to achieve a clear understanding how cocaine affects the brain to produce its pleasurable effects and the reason it is so addictive.
Scientists have found regions of the brain that seem to be stimulated by all kinds of stimuli—food, sex, and drugs of abuse. One of these regions most affected by cocaine is the ventral tegmental area (VTA) in the midbrain.
The way the brain normally functions, research has found, is by nerve fibers in the VTA extending to another region of the brain called the nucleus accumbens, a key region of the brain involved in reward.
Normal Brain and Dopamine Function
Rewards increase levels of dopamine, a brain chemical or neurotransmitter, which in turn increases neural activity in the nucleus accumbens. Under normal circumstances, dopamine is released by a neuron into the small gap between the neurons (synapse) where it then binds to specialized proteins, known as dopamine receptors, on the other neuron, sending a signal to that neuron.
After the signal is sent, dopamine is removed from the gap between the neurons and is recycled for use in the future.
Reward System Amplified
Science has discovered that cocaine and other drugs of abuse can interfere with this normal communication process in the brain. Cocaine use blocks the removal of dopamine from the synapse causing an 'amplified' signal being sent to the receiving neurons.
This amplified signal is what cocaine users perceive as an initial euphoria or high.
But after that initial high, a neurochemical rebound takes place in the brain that causes the reward function to drop below its original normal level. When cocaine is used again, the same level of euphoria is not achieved.
This phenomenon produces a tolerance for the drug in the user, meaning they need higher doses or more frequent doses for the brain to try to achieve the same level of pleasure experienced during their initial use. This cycle of increasing cocaine doses to get the same high can produce an addiction.
Pathological Pursuit of Rewards
Cocaine users develop a tolerance to the 'high' they get by using the drug, but they do not develop a tolerance to the emotional low they feel after the high wears off. Consequently, rather than turning to a state of 'normal,' they revert to a deeper state of dysphoria.
Therefore, they increase the amount of cocaine they use to try to ease that feeling of dysphoria and try to get back to that initial feeling of euphoria. However, they end up experience even deeper lows as the brain reacts to the cycle of intoxication and withdrawal.
That is the point at which the American Society of Addiction Medicine (ASAM) says that the pursuit of rewards becomes pathological and reward-seeking becomes compulsive in spite of the fact that the 'high' is no longer pleasurable and the drug does not provide any relief from dysphoria. Ecomstation 2.2 iso download windows 7.
Prolonged or chronic use of cocaine plays such havoc with the brain's natural reward system to the point that using cocaine no longer produces its initial pleasurable effects.
What Are The Short-Term Effects of Cocaine Use?
Almost immediately after taking cocaine, the user begins to feel its effects, whether it is snorted, injected or smoked. Even small doses of the drug can make the user feel euphoric, energetic, talkative, and mentally alert.
Users report a heightened sensitivity to sight, sound, and touch. They can also experience a decreased need for food or sleep, at least temporarily.
Although some cocaine users find that using the drug helps them perform simple intellectual and physical tasks more quickly, other users report that cocaine has the opposite effect.
The method by which cocaine is used can affect how high the user feels and how long the high lasts. For example, snorting cocaine does not produce as intense a high as smoking it, but the high lasts longer. A high from snorting may last 15 to 30 minutes, while a high from smoking cocaine might last only 5 to 10 minutes.
The faster the drug is absorbed into the bloodstream, the more intense the high, but the shorter the duration.
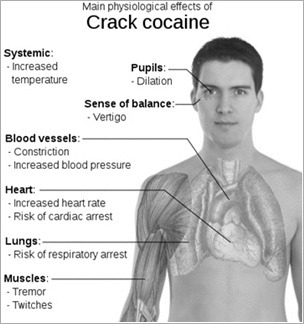
Short-term physiological effects of cocaine can include:
- Constricted blood vessels
- Dilated pupils
- Increased body temperature
- Increased heart rate
- Increased blood pressure
Users who take large amounts of cocaine might intensify their high but may experience bizarre, erratic, and violent behavior. They may also experience:
- Tremors
- Vertigo
- Muscle twitches
- Paranoia
- Restlessness
- Irritability
- Anxiety
According to the National Institute on Drug Abuse, repeated doses of cocaine can produce a toxic reaction closely resembling amphetamine poisoning.
Although it is rare, sudden death can occur on the first use of cocaine or unexpectedly with later doses of the drug. Cocaine-related deaths are often a result of cardiac arrest or seizures followed by respiratory arrest.
Alcohol Increases Cocaine Dangers
Some cocaine users report the drug gives them a feeling of power and confidence. Many times they think they are functioning on a higher level than they actually are. Therefore, driving while doing cocaine can be dangerous especially if you are drinking also.
When drinkers are doing cocaine they have a tendency to drink more than usual because they don't experience the depressant effects of alcohol because of cocaine's stimulant properties. However, when the effect of the cocaine begins to wear off, the drinker is left more intoxicated than he realized, increasing the risk not only of accidents but vomiting, slowed respiration and possible loss of consciousness.
When cocaine and alcohol are used together, they are combined in the liver to form cocaethylene, which intensifies the euphoric effects of cocaine. But, it also increases the strain on the heart and the risk of sudden death.
Withdrawal Symptoms
As the effect of cocaine begins to wear off, you can experience a number of withdrawal symptoms, including irritability, aggression, restlessness, anxiety, insomnia, depression or paranoia.
Because of these unpleasant withdrawal symptoms, many cocaine users report difficulty in 'coming down' from the drug. Many users report depression immediately after the drug's effects wear off, which for some can last for days.
Consequently, some users will take more cocaine to avoid the unpleasant withdrawals—another reason cocaine is considered so highly addictive.
Do you think you may need treatment for drug abuse? Take the drug abuse treatment screening quiz to find out.
What Are the Long-Term Effects of Cocaine Use?
One of the most dangerous consequences of using cocaine is its powerful addictive qualities. Even after one use of the drug, users are not reliably able to predict or control how much he or she will continue to use cocaine or want to use it.
Once someone becomes addicted to cocaine, quitting without relapse become extremely difficult, even after long periods of abstinence. National Institute of Drug Abuse research has shown that even after not using cocaine for long periods of time, exposures to triggers associated with cocaine—or even memories of past cocaine experiences—can set off tremendous cravings and relapses.
When cocaine users continue to use the drug, the brain begins to change its reward system. A tolerance to the drug can develop, meaning that higher or more frequent doses of cocaine is needed to produce the high experienced on initial use.
At the same time, users can become more sensitive to cocaine's anxiety-producing, convulsant and other toxic effects.
Psychological and Physiological Effects
With repeated cocaine binges, when the drug is used repeatedly at increasingly higher doses, the user can risk adverse psychological and physiological effects, including:
- Increased irritability
- Restlessness
- Full blown psychosis involving paranoid delusions and hallucinations
The method by which cocaine is used can produce specific adverse effects. Snorting cocaine can lead to:
- Loss of the sense of smell
- Nosebleeds
- Problems swallowing
- Hoarseness
- Irritation of the nasal septum
- Chronic inflamed, runny nose
Ingesting and Injecting Cocaine
Users who ingest (chew) cocaine can experience severe bowel gangrene due to reduced blood flow.
Those who inject cocaine with needles can develop 'tracks' on their forearms and other injection areas. They can also develop allergic reactions, both to the cocaine itself or to additives used to cut the drug by street dealers.
According to the NIDA, many chronic cocaine users lose their appetite and experience significant weight loss and show signs of malnourishment.
More Long-Term Effects
There are other long-term effects of using cocaine over a period of time. Some of them include:
- Irregular heartbeat, heart attack, and heart failure
- Neurological incidents, including strokes, seizures, and hemorrhaging in tissue surrounding the brain
- Sleeplessness
- Sexual dysfunction
- Perforated nasal septum
- Fluid in the lungs, aggravation of asthma and other lung disorders, and respiratory failure
- Increased risk of traumatic injury
- Aggressive, violent, or criminal behavior
- Increased risk of hepatitis, HIV infection, endocarditis, and fungal brain infections (for IV users)
What Are the Medical Complications of Cocaine Abuse?
The use of cocaine can produce extensive and massive medical complications, the most frequent of which are cardiovascular effects, including disturbances in heart rhythm and heart attacks.
Cocaine use can cause such respiratory effects as chest pain and respiratory failure; neurological effects, including strokes, seizure, and headaches; and gastrointestinal complications, including abdominal pain and nausea.
The repeated use of cocaine has been linked to many types of heart disease. Cocaine has been found to trigger chaotic heart rhythms, called ventricular fibrillation; accelerate heartbeat and breathing; and increase blood pressure and body temperature. Physical symptoms may include chest pain, nausea, blurred vision, fever, muscle spasms, convulsions, and coma.
Adverse Effects of Snorting Cocaine
The different ways that cocaine is used can produce different adverse effects. Regularly snorting cocaine, for example, can lead to loss of sense of smell, nosebleeds, problems with swallowing, hoarseness, and an overall irritation of the nasal septum, which can lead to a chronically inflamed, runny nose.
Ingested cocaine can cause severe bowel gangrene, due to reduced blood flow. And, persons who inject cocaine have puncture marks and 'tracks,' most commonly in their forearms.
Injection Dangers of Cocaine
Users who inject cocaine may also experience an allergic reaction, either to the drug or to some additive in street cocaine, which can result, in severe cases, in death. Because cocaine has a tendency to decrease food intake, many chronic cocaine users lose their appetites and can experience significant weight loss and malnourishment.
For intravenous (IV) cocaine users, there is, of course, an increased risk of hepatitis, HIV infection, and endocarditis.
Hazards of Cocaine and Alcohol
Research has shown a potentially dangerous interaction between cocaine and alcohol. Taken in combination, the two drugs are converted by the body to cocaethylene. Cocaethylene has a longer duration of action in the brain and is more toxic than either drug alone.
While more research needs to be done, it is noteworthy that the mixture of cocaine and alcohol is the most common two-drug combination that results in drug-related death.
Are Cocaine Abusers at Risk for HIV/AIDS and Hepatitis?
Cocaine users are at greater risk for contracting infectious diseases, including human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) and viral hepatitis.
Sharing contaminated needles and other drug paraphernalia is one cause for the increased risk, but also because intoxicated drug users are more likely to engage in risky behaviors.
National Institute on Drug Abuse research shows that drug use and addiction compromises judgment and the ability to make decisions, which can lead to needle sharing, risky sexual encounters, and trading sex for drugs—by both men and women.
Cocaine and HIV and Hepatitis C
The role of sexual transmission of HIV in drug users has been brought to light by some studies that have shown that those drug abusers who do not inject drugs are contracting HIV at rates equal to those who are injection drug users.
Injection drug users are also at increased risk for contracting hepatitis C (HCV). NIDA research shows that the risk of contracting HCV begins with the very first drug injection. Within two years 40% of injection drug users are exposed to the virus and by five years the risk rises to between 50% and 80%.
The NIDA recommends HCV testing for any patient who has ever injected drugs.
What Is the Effect of Maternal Cocaine Use?
Scientists have not been able to determine the complete effect that cocaine use by a pregnant woman has on her child, but studies have found some common risks. Babies whose mothers abused cocaine while pregnant are often:
- Prematurely delivered
- Have low birth weights
- Have smaller head circumferences
- Shorter in length
One reason that researchers have not been able to determine the full extent of maternal drug abuse or specific hazards of cocaine on an unborn child is because if the mother is abusing cocaine, there is likely that other factors may be at play in her life that could also affect the baby.
Other Factors Play a Role
Some of the other factors that could impact maternal, fetal and child outcomes include:
- Amount and number of drugs abused
- Use of nicotine
- Extent of prenatal care
- Violence in the environment
- Socioeconomic conditions
- Maternal nutrition
- Exposure to sexually transmitted diseases
- Other health conditions
General Effects Of Crack Use Include Burning Of The
Cognitive Effects on Baby
Other consequences of prenatal cocaine abuse that researchers have been able to identify include deficits in some aspects of information processing, attention to tasks, and cognitive performance. All of these deficits could hamper the child's achieving his or her full potential, the National Institute on Drug Abuse said.
What Treatments Are Effective for Cocaine Abusers?
Cocaine addiction can be a complex condition, causing the addict issues not only with the addiction itself but with a wide variety of personal problems. Treatment for cocaine, therefore, needs to be a comprehensive approach to addressing the addict's social, family and other environmental problems.
According to the National Institute on Drug Abuse, cocaine treatment strategies need to include assessment of the neurobiological, social, and medical aspects of the patient's drug use. Many times this includes multiple drugs of abuse.
Additionally, those who are addicted to multiple drugs also often have other co-occurring mental health issues which also must be addressed in treatment.
Pharmacological Approaches
There are currently no medications approved by the U.S. Food and Drug Administration to treat cocaine addiction, although aggressive research is being conducted to find and test new medications that can help cocaine addicts.
General Effects Of Crack Use Include Burning The Body
Some of the medications currently being tested are those that are FDA approved for other conditions or diseases. Some that are showing promise for cocaine treatment include vigabatrin, modafinil, tiagabine, disulfiram, and topiramate.
New medications are being researched that block the effects of cocaine on various areas of the brain to help prevent relapse in patients who have already quit using the drug. This includes a 'cocaine vaccine' that has shown 'great promise,' the NIDA says.
Behavioral Interventions
There are several behavioral treatments that are being used in residential and outpatient settings to treat cocaine addictions. Currently, they are the only approved and evidence-based treatments available for cocaine and crack cocaine abusers.
- Motivation incentives (contingency management)
- Therapeutic communities (residential programs)
- Support groups (such as Cocaine Anonymous)
- Allen, Frederick.Secret Formula. New York: HarperCollins, 1994. ISBN 0-88730-672-1 (pp. 35-36, 41-42, 45, 192).
- American Society of Addiction Medicine. 'The Definition of Addiction (Long Version).' 15 August 2011.
- Brown University. 'Cocaine.'Health Promotion - Alcohol, Tobacco, & Other Drugs.
- National Institute on Drug Abuse. 'Cocaine: Abuse and Addiction.'Research Report Series.
- University of Maryland Center for Substance Abuse Research. 'Cocaine (Powder).'Drug Information.