Bcs Classification Of Drugs List
Essential drugs, drug databases of Combined List (346 drugs), Western List (147 drugs), Eastern List (163 drugs) was made and analyzed on molecular properties and BCS classification. GUIDANCE ON BIOPHARMACEUTICS CLASSIFICATION SYSTEM (BCS)-BASED BIOWAIVER National Pharmaceutical Control Bureau, Ministry Of Health Malaysia. January 2013 Adopted and adapted mainly from the following: 1. Guideline On The Investigation Of Bioequivalence (European Medicines Agency, London, 20 January 2010, CPMP/EWP/QWP/1401/98 Rev. Durga amritvani song download free.
 Dec 23, 2018 - Boruto Episode 87 Subtitle Indonesia. Download Boruto 87 Sub Indo. Silakan kunjungi di RiiE.net untuk nonton streaming. Eps 88 selanjutnya. Apr 17, 2018 - Nonton streaming Boruto subtitle Indonesia: download anime sub Indo. Boruto Episode 89 Subtitle Indonesia Download Boruto 89 Sub Indo Silakan kunjungi di RiiE.net untuk nonton streaming Eps 90 selanjutnya rilis tanggal 20. DOWNLOAD BORUTO: NARUTO NEXT GENERATIONS SUBTITLE INDONESIA. By Anime Naruto Indonesia Updated about 4 months ago Taken in.
Dec 23, 2018 - Boruto Episode 87 Subtitle Indonesia. Download Boruto 87 Sub Indo. Silakan kunjungi di RiiE.net untuk nonton streaming. Eps 88 selanjutnya. Apr 17, 2018 - Nonton streaming Boruto subtitle Indonesia: download anime sub Indo. Boruto Episode 89 Subtitle Indonesia Download Boruto 89 Sub Indo Silakan kunjungi di RiiE.net untuk nonton streaming Eps 90 selanjutnya rilis tanggal 20. DOWNLOAD BORUTO: NARUTO NEXT GENERATIONS SUBTITLE INDONESIA. By Anime Naruto Indonesia Updated about 4 months ago Taken in.
- Docket Number:
- FDA-2015-D-1245
- Issued by:


The Food and Drug Administration (FDA or Agency) is announcing the availability of a guidance for industry entitled “Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System.” This guidance finalizes recommendations for sponsors of investigational new drug applications (INDs), and applicants who submit new drug applications (NDAs), abbreviated new drug applications (ANDAs), and supplements to these applications for immediate-release (IR) solid oral dosage forms, and who wish to request a waiver of an in vivo bioavailability (BA) and/or bioequivalence (BE) study requirement.
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Division of Dockets Management (HFA- 305)
Food and Drug Administration
5630 Fishers Lane, Rm. 1061
Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2015-D-1245.
Regulated Product(s)
Bcs Classification Of Drugs List Who
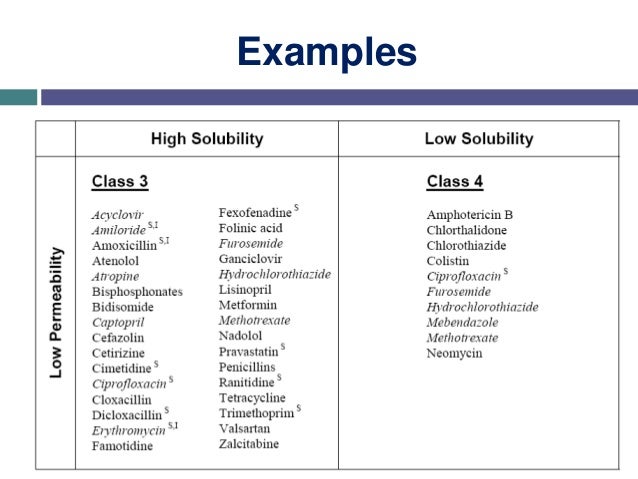
BCS Divides Compounds into Four Categories:
Class I – High Solubility, High Permeability
Class II – Low Solubility, High Permeability
Class III – High Solubility, Low Permeability
Class IV – Low Solubility, Low Permeability
Mar 1, 2018 - Picktorrent: panic at the disco discography - Free Search and Download Torrents at search engine. Download Music, TV Shows, Movies, Anime. Panic at the disco discography torrent download windows 7.
Furthermore, for Class I compounds, it is unlikely that absorption will be limited by efflux transporters. Thus, it may be possible to waive clinical DDI studies as well.“If in vitro experiments demonstrate that an NME is a P-gp substrate, additional drug-specific factors may be considered before determining whether an in vivo drug interaction study is warranted. For example, the bioavailability of the BCS Class I or BDDCS Class I NMEs that are highly soluble, highly permeable, and highly metabolized may not be significantly affected by a co-administered drug that is a P-gp inhibitor, and thus, an in vivo interaction study may not be needed.”
— L. Zhang et al., “Predicting Drug-Drug Interactions: An FDA Perspective” (2009) The AAPS Journal
Absorption Systems, World Leader in Biopharmaceutics Classification System
Absorption Systems has performed dozens of BCS classification studies over the past ten years, many of which have resulted in successful BCS biowaiver submissions. Eight of the ten largest generic drug companies have conducted in vitro BCS biowaiver studies with Absorption Systems. It was our permeability data that supported some of the very first in vitro BCS biowaivers.
Our extensive experience continues to facilitate innovative solutions and enable in vitro classification for a broader range of drugs. This, combined with our 10-day turnaround for pre-qualification and our cost effective designs, saves you time and money. Just a few reasons why Absorption Systems averages more than 30 BCS studies each year.
Avoid expensive and time consuming clinical bioequivalence studies with in vitrosolubility, permeability, and dissolution data. It is a critical optimizer in time and money for drug developers.
Our validated 3-step study design allows for early identification of BCS biowaiver candidates and optimization of protocol elements. These steps include:
- Pre-qualification and determination of the eligibility of test compound for BCS biowaiver – your go/no-go decision point
- Protocol optimization and conduct of FDA-required experiments to establish protocol for pivotal studies
- GLP BCS classification of permeability and/or solubility